Revealing the secrets of glass formation
School researcher Dr James Drewitt is part of a team that has - for the first time - obtained a complete picture of the in situ structure of fragile glass-forming liquids at very high temperature. The results are published in Physical Review Letters [1].
The glassy state is ubiquitous in nature and technology. However, their structural complexity and the high temperatures involved in the formation of glasses mean that surprisingly little is known about the structural processes that take place when glasses are formed. Here James Drewitt describes the work behind the team's results.
Glasses have a disordered atomic structure, bearing similarities to that of a liquid. They are formed by supercooling a liquid, ie lowering the temperature below its freezing point without it solidifying, until its atomic structure is frozen into the solid state at its glass transition temperature. In the present work we have studied a "fragile" glass former. Unlike traditional "strong" network glass formers such as SiO2, the viscosity and other dynamical properties of fragile liquids experience a dramatic slow-down with decreasing temperature upon glass formation. It is therefore interesting to know how this viscous slow-down may be related to changes in the atomic structure.
Calcium aluminates
Molten calcium aluminates are very fragile glass-forming refractory liquids. Refractory materials retain their strength and structural integrity at high temperatures leading to their application as furnace linings or high temperature crucibles. They also form a significant component of natural magmas, and have a diverse range of other applications including integral components of aluminous cements (valued for their high resistance to temperature and chemicals), and bioceramics (used in medical procedures). The glasses have optoelectronic applications in the infra-red spectrum, enabling their use in infra-red optical fibres, and the luminescent properties of glasses containing rare earth ions make them suitable for applications such as pre-amplifiers and solid-state lasers.
Experimental technique
A liquid and glass of composition CaAl2O4 were investigated using neutron diffraction with 44Ca isotope substitution using the D4c neutron diffractometer at the Institut Laue-Langevin in Grenoble, France (figure 1). The small (2.5 mm diameter) liquid droplets were aerodynamically levitated by suspension in a stream of argon gas and heated by infra-red laser beams. The experimental information was interpreted with the aid of molecular dynamics computer simulations.
Unexpected structural changes
The results reveal dramatic and unexpected changes in the material’s structure during glass formation. In the liquid, the aluminium atoms form AlO4 and AlO5 structural motifs, and there are significant numbers of threefold coordinated oxygen atoms in so-called tricluster conformations where a single oxygen atom is shared between three AlO4 tetrahedra.
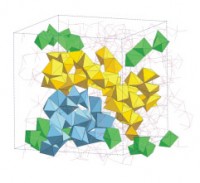
During glass formation, the AlO5 units and threefold coordinated oxygen atoms break down as the structure reorganises to form a network of predominantly corner-sharing AlO4 tetrahedra. This process is accompanied by the formation of branched chains of edge- and face-sharing Ca-centred CaOx polyhedra that weave through the glass network (figure 2).
Conclusion
Glasses are often used as analogues for understanding liquids of geophysical importance under high pressure and temperature conditions. Calcium aluminosilicates, for example, constitute one of the main fractions of the composition of the Earth's mantle, and detailed knowledge of the structure and properties of their melts is important for understanding processes involving the presence of magmas at depth.
However, the new results show the process of glass formation in materials such as calcium aluminate is accompanied by a substantial change in structure, demonstrating there is no realistic substitute to in situ diffraction experiments to determine the melt structure. The results from [1] for the high-temperature liquid will also provide a benchmark for interpreting the structural changes that occur when high pressures are applied.
[1] James W E Drewitt, Louis Hennet, Anita Zeidler, Sandro Jahn, Philip S Salmon, Daniel R Neuville, Henry E Fischer, Physical Review Letters 109 235501 (2012).
The experimental work was performed at the Institut Laue Langevin (ILL) utilising the levitation device developed at the CEMHTI-CNRS lab in Orléans. The experimental team included James Drewitt (Edinburgh, UK), Louis Hennet (CEMHTI, Orléans), Anita Zeidler and Phil Salmon (Bath, UK), Daniel Neuville (IPGP, Paris), and Henry Fischer (ILL, Grenoble). The molecular dynamics simulations were made by Sandro Jahn (GFZ, Potsdam).

